Research Interests
My research focuses on understanding the mechanisms that drive the interplay between various scales of biological organisation and their impact on concurrent ecological and evolutionary dynamics. I use modelling approaches to understand how microscopic processes (such as gene-regulatory network architecture) impact macro-scale ecological dynamics (e.g. range expansions) via their effect on evolutionary potential, and also how macro-scale ecological context such as landscape structure impacts the evolution of traits governing species interactions and spread (e.g., co-evolution of host dispersal and parasite virulence in host-parasite systems in complex landscapes).Conceptual problems in eco-evolutionary dynamics and dispersal evolution
I am interested in conceptual issues around emergence in eco-evolutionary dynamics specifying the conditions (e.g., time scales, genetic architecture, magnitude of ecological change) under which it is relevant to consider the interplay between ecological and evolutionary change. I have collaborated on a perspectives article (Fronhofer et al., 2023, ELE) that explores this in detail. My modelling work (Deshpande and Fronhofer, 2022, PNAS) also contributes to this area by highlighting the role of evolution (genetic architecture) in ecological (range expansion) dynamics. In a similar vein, I am interested on the causes and consequence of dispersal evolution in multi-species systems, focusing on antagonistic species interactions (Zilio et al., 2024, TREE, accepted).
Eco-evolutionary dynamics of host-parasite systems in complex spatial networks.
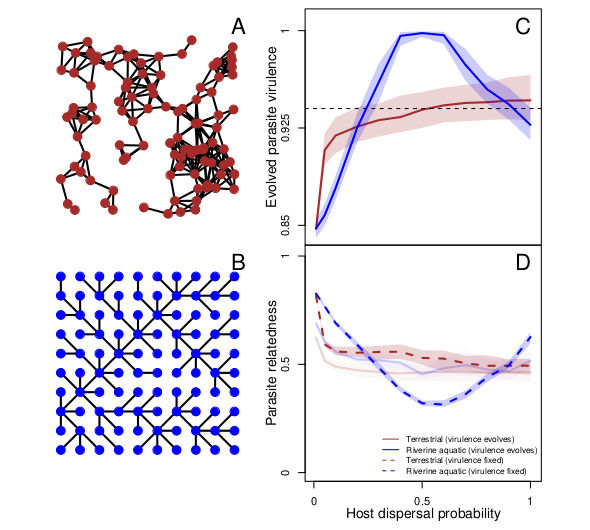
The interactions between species in ecological systems (e.g. plant-pollinator, foodwebs and host-parasite systems) happen within a spatial context which is modelled by spatial networks. The topology of these spatial networks can impact ecological and evolutionary dynamics, and how they feedback onto each other by distributing organisms and genetic material. Focusing on host-parasite systems, I seek to understand how the spatial context of host-parasite systems, impacts the evolution of traits governing their interactions and their feedback on epidemiological dynamics. In Deshpande et al. (2023, bioRxiv, under review), using models, we showed that terrestrial and riverine aquatic landscapes should lead to diverging patterns of virulence evolution in the two landscape types. Within this framework, I am also interested in the co-evolution of host dispersal and parasite virulence terrestrial vs. aquatic landscapes.
Impact of genetic architecture of dispersal and local adaptation on range expansion dynamics.
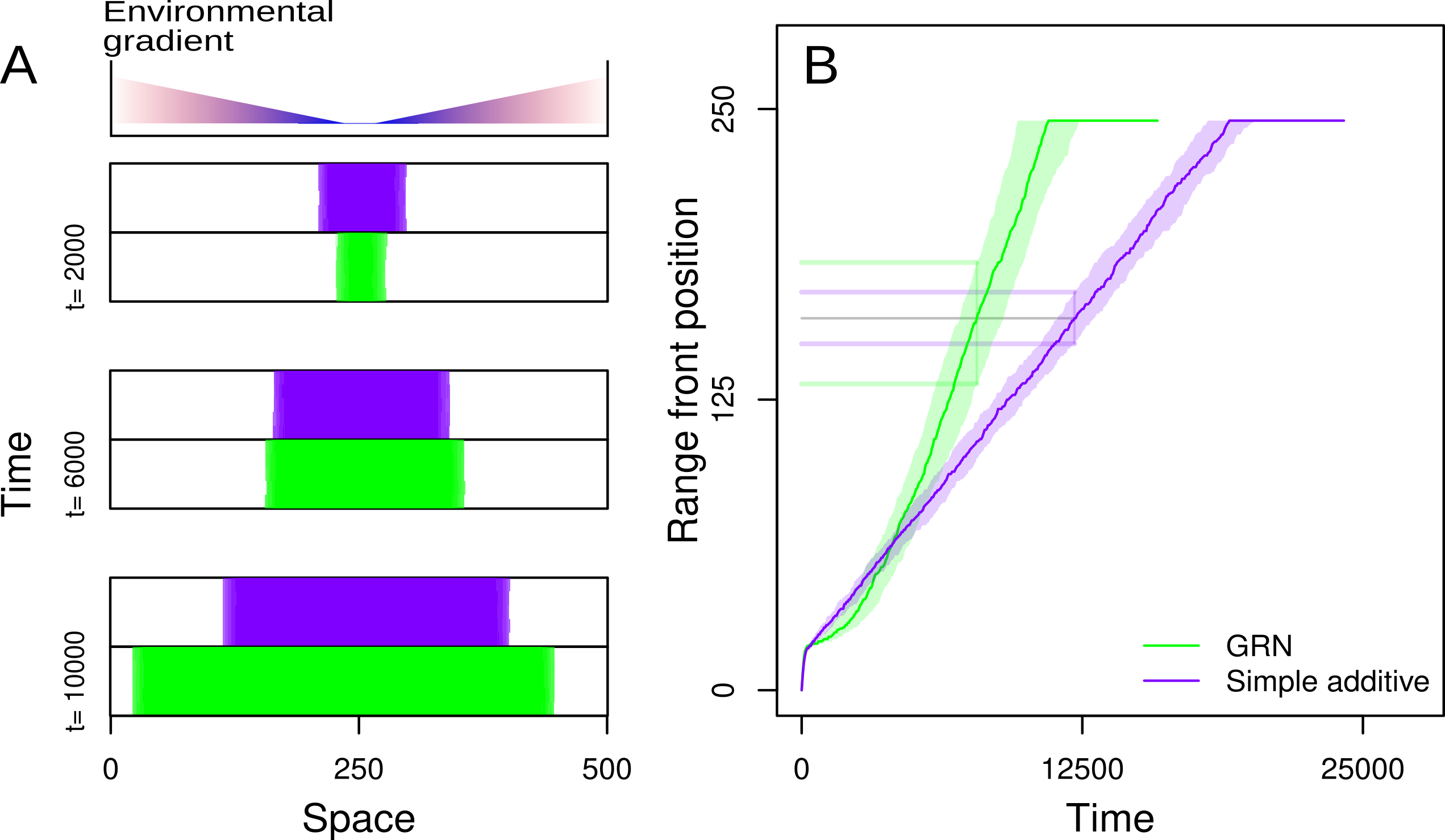
When ecological and evolutionary change occurs on similar timescales, eco-evolutionary feedbacks not only depend on long term evolutionary optima but also on the rate of evolutionary change (evolvability). The rate of evolutionary change is thus strongly impacted by both the amount of genetic variation which is the raw material for evolution, and also processes that generate this variation, which depend on the genetic architecture of traits. In this context, in collaboration with Emanuel A. Fronhofer, I explored how the genetic architecture of dispersal and local adaptation traits inpact the speed of range expansions. In Deshpande & Fronhofer (2022, PNAS), we showed using models that assuming a gene-regulatory network (GRN) genetic architecture as opposed to simple additive genetics, leads to accelerating range expansions into an external environmental gradient. I also studied how gene-regulatory networks can be used to model dispersal plasticity, particularly density-dependent dispersal and its consequences on range expansion dynamics (Deshpande & Fronhofer, 2023, bioRxiv, under review).
Evolution of state and context-dependent dispersal in host-parasite systems.
Organisms often receive internal and external cues to inform their dispersal decisions, this is known as dispersal plasticity. In a host-parasite context, hosts may receive internal cues on their infection state and external cues on disease prevalence. In collaboration with Emanuel A. Fronhofer and Oliver Kaltz, we showed in Deshpande et al. (2021, Oikos) how infection state- and prevalence-dependent host dispersal evolves in host-parasite systems and the mechanisms driving its evolution.